Products
STRUCTURAL BONE
BACKFILL PLUGS
Cancellous backfill plugs, designed for use with the COR® Precision Targeting System, are used to fill autograft harvest sites created during the repair of osteochondral defects. The osteoconductive backfill plugs act as a scaffold for the growth of natural bone and may reduce the risk of donor site morbidity. The backfill plugs are gamma-irradiated in their final packaging to achieve an SAL of 10-6.
BONE DOWELS
Allograft bone dowels are commonly used for ACL revision tunnel backfill. The cylindrical graft with cortical face, cancellous body, and a pilot hole is designed to provide an immediate scaffold that restores the open void and promotes natural bone growth. The bone dowels are gamma-irradiated in their final packaging to achieve an SAL of 10-6.
Parametrics Medical is the exclusive biologics provider for J&J MedTech.
FASCIA LATA
FASCIA LATA
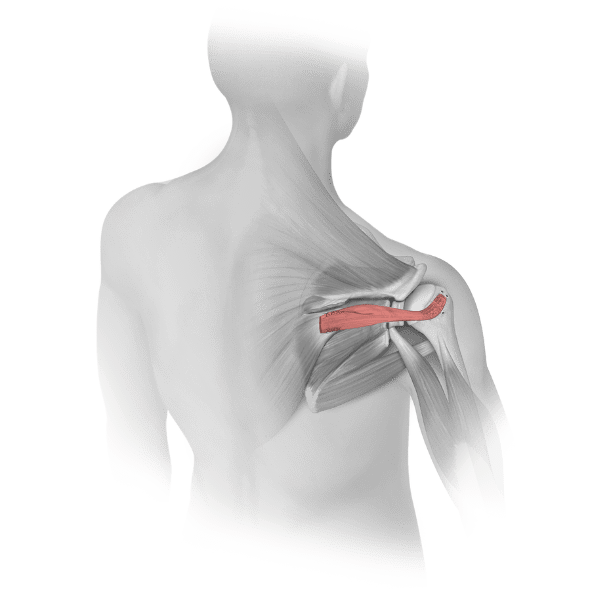
Fascia Lata is a fibrous covering of muscle recovered from the lateral aspect of the thigh used in various clinical applications such as periodontal surgery, dural defect repair, hip labral reconstruction, and superior capsule reconstruction. These allografts are gamma-irradiated in their final packaging to achieve an SAL of 10-6.
Parametrics Medical is the exclusive biologics provider for J&J MedTech.
CARTILAGE
CARTILAGE
DISTAL TIBIA WITH CARTILAGE
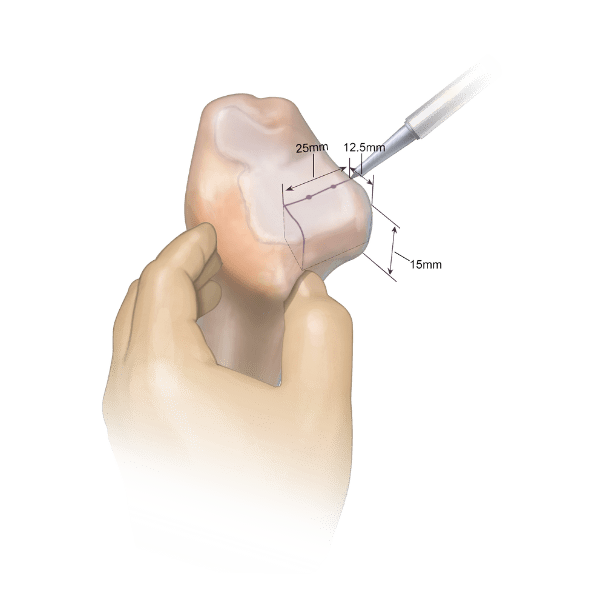
Distal tibia allograft may be used to treat shoulder instability due to glenoid bone loss. The distal tibia’s lateral portion matches the patient’s glenoid because it has similar curvature, bone density, and cartilage.
| Product Number | Description |
|---|---|
| OSTD2070ROA | Fresh OA Tibia, Distal, Right |
| OSTD2070LOA | Fresh OA Tibia, Distal, Left |
| OSTD2070RFC | Distal Tibia w/Cartilage, Right, Frozen, Irradiated |
| OSTD2070LFC | Distal Tibia w/Cartilage, Left, Frozen, Irradiated |
MENISCUS
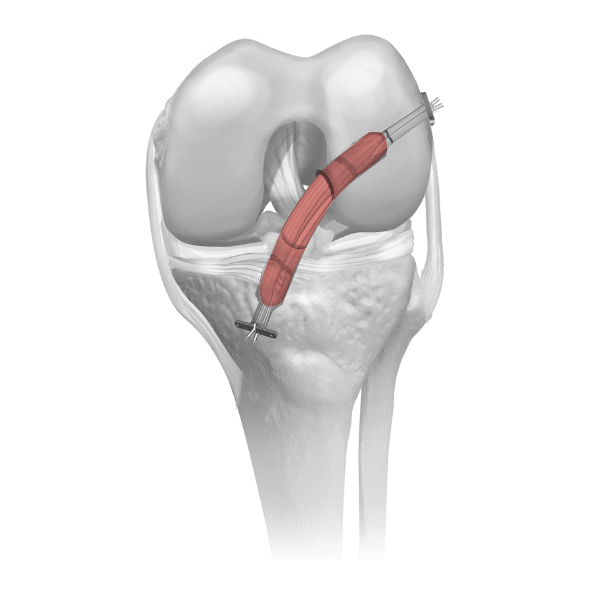
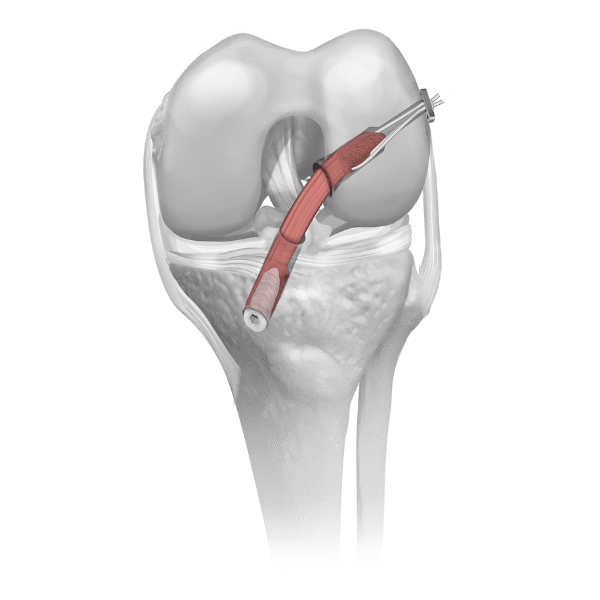
The biomechanical role of the meniscus is load distribution and stability in the knee joint. Parametrics Medical’s allograft meniscus with hemi plateau bone block is used in meniscal allograft transplant procedures and one of the few viable treatment options for the young meniscectomized knee. Our allograft meniscus is aseptically processed. No irradiation is used in processing or after packaging.
Parametrics Medical is the exclusive biologics provider for J&J MedTech.
ALLOGRAFT TENDONS
ALLOGRAFT TENDONS
- No donor site morbidity
- Reduced surgical time
- Available in various sizes to meet surgeon preference
Parametrics Medical offers three unique processing options for allograft tendons (Coll-e-Strong™, Lo-RAD™, and No-RAD™). Most tissue banks only provide one processing option.
No-RAD allografts are Parametrics Medical’s trademarked aseptically processed tendons. No irradiation is used in processing or after packaging. The No-RAD processing methodology achieves an SAL of 10-3.
Parametrics Medical is the exclusive biologics provider for J&J MedTech.
ACELLULAR DERMIS
ACELLULAR DERMIS
DERMIS ON DEMAND™ ALLOGRAFT
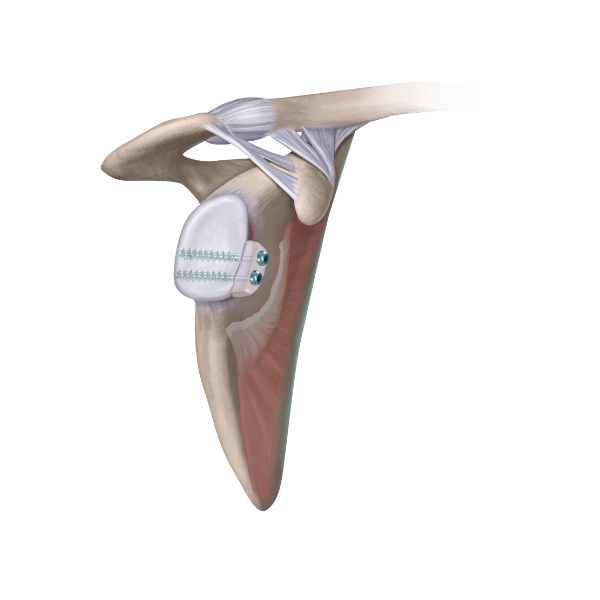
Dermis on Demand (DOD) is a shelf-stable human allograft patch intended for supplemental support, protection, reinforcement, or soft tissue covering. DOD may be used in numerous clinical applications, including but not limited to: rotator cuff repair, lateral ankle stabilization, and hip labral repairs.
- DOD provides a broader compression area than suture tape alone, which may correspond with more tendon-to-bone contact.
- DOD is easy to use and engineered for placement in 30 seconds. DOD is pre-packaged with a threader for easy suture loading.
- DOD features OPEN DERMAL MATRIX™ Technology, which has been shown, in pre-clinical animal models, to integrate better than tested traditional dermal allografts.
RESOURCES
Catalogs
Product Brochures
Parametrics Medical is the exclusive biologics provider for J&J MedTech.