ALLOGRAFT TENDONS
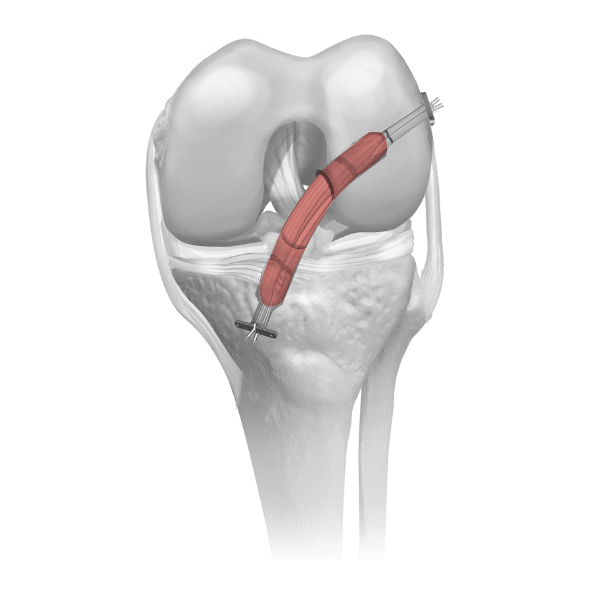
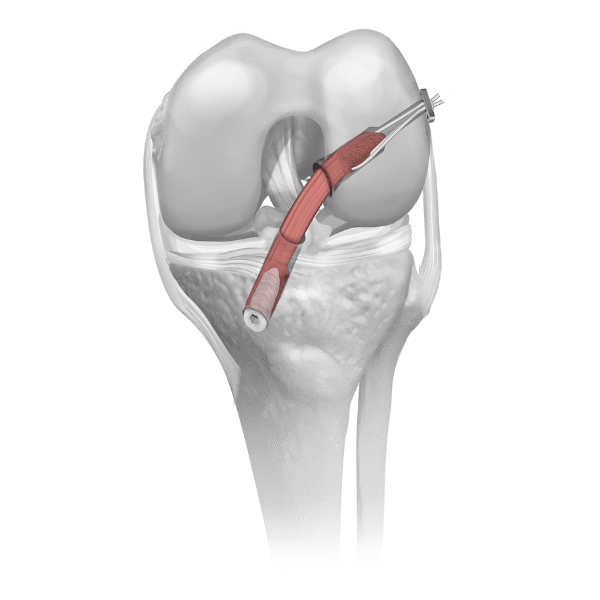
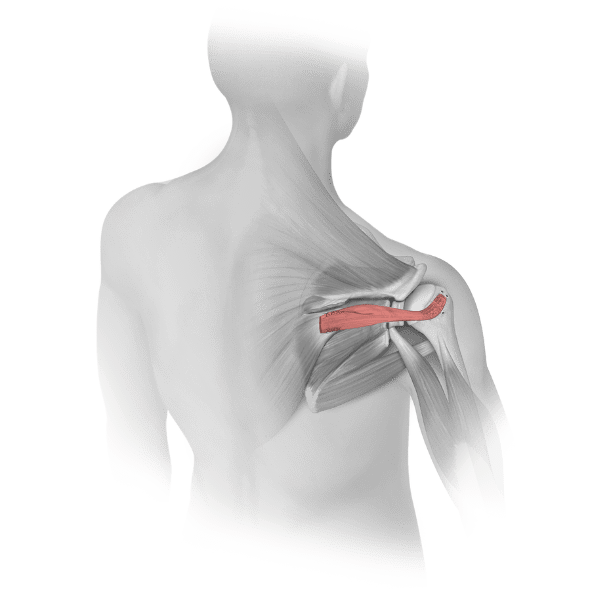
Parametrics Medical offers a complete portfolio of allograft tendons used in numerous clinical applications, including but not limited to: ACL reconstruction, MPFL reconstruction, and lateral ankle stabilization. Allograft tendons (obtained from a donor) are a viable alternative to autograft tendons (obtained from the patient’s body). Benefits of using allograft versus autograft include:
- No donor site morbidity
- Reduced surgical time
- Available in various sizes to meet surgeon preference
Parametrics Medical offers three unique processing options for allograft tendons (Coll-e-Strong™, Lo-RAD™, and No-RAD™). Most tissue banks only provide one processing option.
Coll-e-Strong allografts are Parametrics Medical’s trademarked electron beam sterilized tendons. This innovative processing methodology achieves a sterility assurance level (SAL) of 10-6 without the use of gamma irradiation. Coll-e-Strong grafts provide Parametrics Medical’s best option of sterility, safety, and strength for surgeons and patients.
PRODUCT NUMBERS
Lo-RAD allografts are Parametrics Medical’s trademarked low-dose gamma-irradiated tendons. These allografts are processed with an average exposure rate of 1.25 megarads which is among the lowest level of irradiation on the market. Studies suggest this level of irradiation does not negatively impact clinical results or biomechanical properties. The Lo-RAD processing methodology achieves an SAL of 10-6.
PRODUCT NUMBERS
No-RAD allografts are Parametrics Medical’s trademarked aseptically processed tendons. No irradiation is used in processing or after packaging. The No-RAD processing methodology achieves an SAL of 10-3.
PRODUCT NUMBERS
Parametrics Medical is the exclusive biologics provider for J&J MedTech.