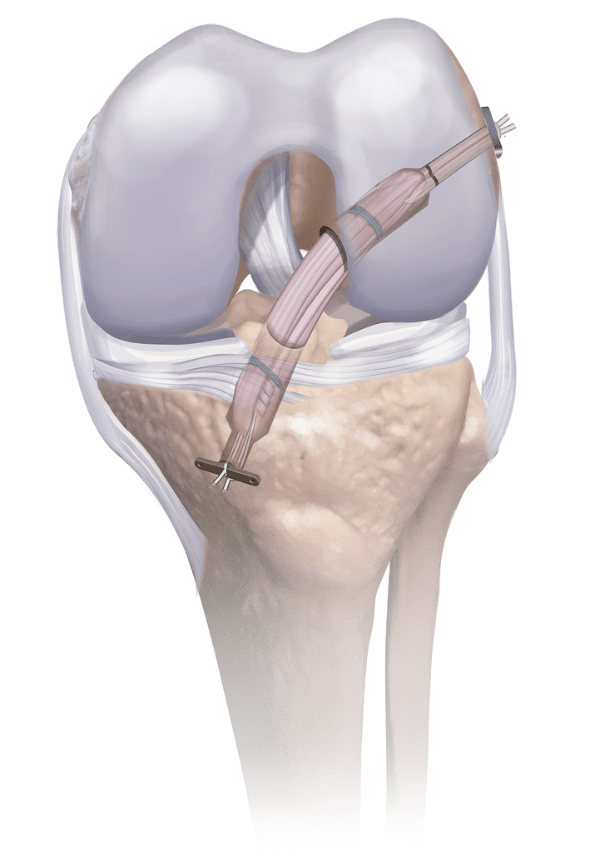
What is an allograft?
An allograft is donated human tissue or bone that has been processed and prepared to aid in the surgical repair of damaged joints, tendons, or bones.
Why use an allograft?
Using allografts eliminates the need for a second surgical site to remove autograft bone or tendons, thus reducing the risk of infection and the pain and/or loss of function from that second surgical site. Allografts come in a variety of sizes, eliminating the guesswork that can come from the size and availability of an autograft. OR time is thereby reduced, as is the cost of the surgery.
Where do allografts come from?
Allografts come from human tissue donors who have generously donated their bodies so that others may benefit.




